RESEARCH OVERVIEW
WHAT WE DO IN THE THOMPSON LAB…
We are developing novel synthetic chemical and biochemical tools to address fundamental problems in human health, with a special emphasis on continuous flow chemistry, delivery of nucleic acid nanoparticles, accelerated protein structure determination using cryo-EM, and lysosomal storage disorder therapeutics. Development of efficient chemoselective routes to these materials is a major focus of our research. We are also exploring the effects of particle shape, size, and environmentally responsive transformations (e.g., pH, enzyme, light, ultrasound) on therapeutic performance. Translation of these basic studies to animal models of disease (e.g., bladder, lung, pancreatic & breast tumors) is the near-term goal of our materials development efforts.
Click on the links or scroll down for a brief description of these research projects. Check out our publications page to learn more about our projects.
RESEARCH TOPICS
Continuous Flow Chemistry
Nucleic Acid Delivery
Accelerated protein structure
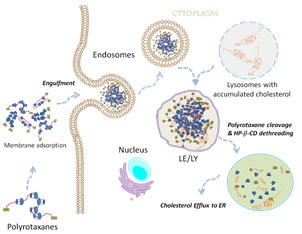
Lysosomal Storage Disorder therapeutics